UMF student Isaiah Wilson-McFarlane ’20 reveals why the summertime monitoring of Maine’s lakes and ponds is vital to their health, our economic vitality, and maintaining The Way Life Should Be.
For this month’s dispatch on the natural splendor of our environs, science writer and photographer Isaiah Wilson-McFarlane ’20 reports on the summertime work of two alumni: veteran water resources technician Ryan Burton ’02 of the Cobbossee Watershed District, and his intern, newly minted graduate Stephanie Dwinal ’19 of Monmouth. In keeping with Wilson-McFarlane’s other writings for Farmington First, you needn’t be steeped in scientific terminology to understand the relevance of their research on the health of central Maine’s lakes and ponds. And by the article’s end, you’ll may well agree that for these three still waters run deep.
— Marc Glass, director of advancement

After moving to Maine from Michigan at the age of 11, Wilson-McFarlane found himself delightfully surrounded by smaller, but more numerous camp-lined lakes, including Rangeley Lake (above), which he photographed recently in late May.
Lakes have always figured prominently in my life. I was born and raised for 11 years in Michigan, always within a stone’s throw of Lake Michigan. I was at the beach every day in the summer heat, looking for sticks to sword fight my brother with or trying to belly surf in the waves. Watching thunderstorms touch the beach was an ever-humbling experience and finding lightning-struck sand — fused into a hollow silica tube and often referred to as “Petrified Lightning” — was my version of Indiana Jones finding the holy grail.
It’s hard to imagine something as impressive and unabating as The Great Lakes that hold a fifth of all freshwater on earth being “sick.” But as I got older and started to grasp that a lake was really thousands of different independent factors all working together to hold equilibrium, I felt a sense of responsibility to understand how they work — to one day keep it from being sick.
After moving to Maine in the summer before 7th grade, I was at the opposite end of the spectrum from my surroundings in Michigan: Instead of seeing one massive lake, I was surrounded by literally thousands of small, beautiful, camp-lined lakes. Many of my friends have family camps near a lake, and it almost feels like part of the Maine identity to spend summer days in a WiFi-less camp, cannonballing off of the dock into iced-tea colored water.
I have also come to realize that this deeply ingrained part of the Maine experience — and large attraction for out-of-staters that arguably allows Maine to claim the mantle of “Vacationland” or “The Way Life Should Be” — doesn’t come for free. It takes a massive scientific effort that for the most part goes unnoticed, behind the scenes. I have been lucky enough through my studies at UMF to have the opportunity to spend hundreds of hours on lakes, researching some of the factors that create this very beautiful, biodiverse, and lucrative inland lake landscape that we all share and benefit from in some form or another no matter how far we may be removed from inland lakes.
To better understand some of the summer science done on Maine’s lakes, I decided to tag along with two UMF graduates to document their work monitoring central Maine’s Cobbossee Watershed.

Intern Stephanie Dwinal ’19 (left) and veteran water resources technician Ryan Burton ’02 of the Cobbossee Watershed District (right) set out for work on Maranacook Lake.
On an overcast June morning, we cradled our gas-station coffees as our boat slid into Maranacook Lake. My good friend Stephanie Dwinal ’19 began organizing gear and monitors, getting ready to leave the dock, while Ryan Burton ’02 parked the truck and trailer before jumping on board. Dwinal is in her second summer working on the lakes that make up the Cobbossee watershed as an intern with the Cobbossee Watershed District. This was just another day for them spent in an aluminum outboard motor boat collecting data, but I was excited to be able to spend the day on a boat doing things I had practiced briefly in my Aquatic Biology class last fall.

Burton drops anchor on Maranacook Lake.
As the motor shut off and the boat rode its own wake to the first data site, a powerful sense of place came over me. Field work is satisfying, testing, and rewarding all at once — something which I can’t forgo for too long without missing.
After dropping anchor, Ryan pulled a Dissolved Oxygen and Temperature meter out of a red box and slowly lowered it into the glass-smooth water. He told me to get comfortable while he meticulously took measurements at every meter of the 123-foot-deep glacially formed lake. In order to understand the complexities of what appears to simply be a low spot in the ground filled with water, think of every single factor of a lake, such as its surface area, depth, native plants and animals, and seasonal changes. A change in any one of these features directly or indirectly alters the rest. This is why regular monitoring is vitally important to understand the health of lakes. Understanding how one single factor can affect the rest allows for informed decisions about maintaining lake health.

Burton begins by taking vertical profile measurements.
Measuring dissolved oxygen is one of the staples of lake quality monitoring, because it indicates so many things about the organisms and the metabolism of a lake. Generally, oxygen is mixed into the lake via both the atmosphere (e.g., wind action) and by photosynthesis by aquatic plants and algae. Bacteria in benthic (lake bed) sediments use oxygen for aerobic decomposition of dead plants and organisms that sink — a vitally important function. These bacteria convert biologically unusable forms of nitrogen into useful forms called nitrates that are necessary for plant growth. More nitrates means more plants.

Dwinal helps guide the boat into Torsey Pond near Readfield, the next stop on the day’s tour within the Cobbossee Watershed District.
But as with most things, a good balance is necessary to avoid problems. Phosphorus sources include, among others, fertilizers from lawns and agriculture and the erosion of watershed soils. When phosphorus from fertilizers spread on lawns and used in agriculture washes into the water, when watershed soils erode into the water, and increasingly warm weather extends the growing season, the resulting algal blooms of cyanobacteria wreak havoc in lake systems. Cyanobacteria generally produce oxygen via photosynthesis. Through nutrient depletion and loss of buoyancy, they die off and contribute to the organic load to the sediments where they are respired by benthic bacteria, resulting in a depletion of dissolved oxygen in bottom waters. This, in turn, feeds future blooms. These cyclical occurrences are biologically damaging, and also have a massive impact on the economy surrounding lakes. No one can swim in the lake, the smell of the water becomes unpleasant, and the clarity of the water is diminished. Not long after these effects, property values decline and, subsequently, so do property tax revenues.

Taking a lake core sample is like sticking a straw into a drink, putting your thumb over the top, and pulling out the straw. The process collects samples from the vertical profile of the lake water.
One means of combating the growth of cyanobacteria involves introducing Alum (aluminum sulfate and sodium sulfate) into lakes and ponds. Just recently Cochnewagon Lake was treated with Alum to minimize the risk of algal blooms. Alum works by trapping nutrient phosphorus (from fertilizer spread on lawns and used in agriculture, as well as manure that reaches the water through soil erosion) in a chemical reaction that converts the aluminum sulfate and sodium aluminate into a substance referred to as floc. The floc that forms during an Alum treatment is from the reaction of the two aluminum chemicals we introduced as liquids. They form the solid precipitate as soon as they interact. The aluminum floc also picks up other matter in the water as it sinks. The aluminum floc does settle on the bottom where it provides adsorption sites for phosphorus, thereby keeping phosphorus tied to the lake sediments, and therefore unavailable for algal growth. This substance is insoluble in water, sinks to the bottom, and cannot be metabolized by algae, which inhibits its growth. Stephanie and Ryan used underwater cameras to follow the floc sinking to the bottom to make sure it went where it was supposed to, as conducted pH tests to make sure that the Alum treatment wasn’t affecting fish.

Burton lowers a black-and-white Secchi Disk into the water to assess zooplankton and phytoplankton concentrations in the lake.
After Stephanie retrieved the Dissolved Oxygen meter and stored it away, she plunked a black- and-white disk attached to a meter tape into the water and slowly lowered it into the dark green water. The Secchi Disk shows the productivity of the water column by indirectly noting zooplankton and phytoplankton concentrations. The farther down into the water you can see the black and white panels of the Secchi Disk, the less zooplankton and phytoplankton, as well as suspended inorganic particles, are present in the water. If you scoop a handful of lake water and look at it under a microscope, you would see hundreds of microscopic copepods and algae that form the base of the food chain. Many fish depend on these zooplankton to live, making it an extremely important part of the lake ecosystem. Earlier springs and later autumns can cause a disconnect between zooplankton population increase and fish spawn that need a large stable zooplankton community as a food source to support their growth.
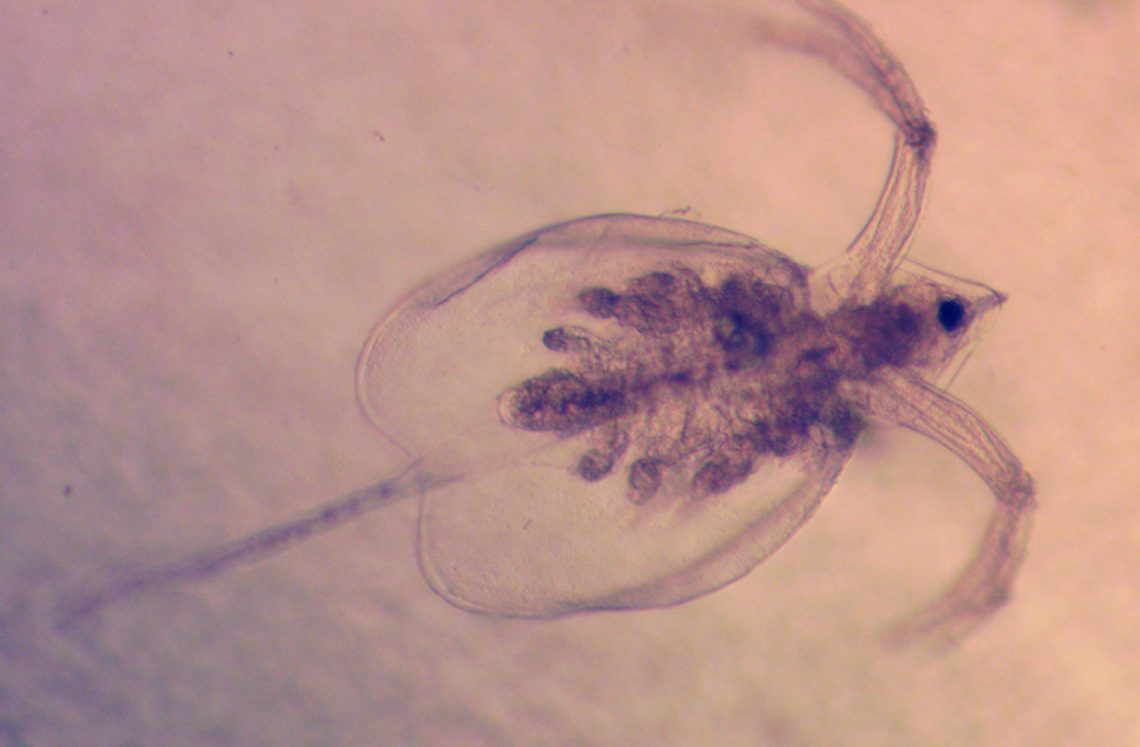
I took this image of zooplankton with a camera mounted to a microscope I was using to count the organisms for my internship with professors Rachel Hovel and Julia Daly.
In other instances, what’s known as a bottom-up trophic cascade can occur when cyanobacteria blooms block sunlight that allows phytoplankton to grow. This, in turn, reduces the food that feeds zooplankton in the lake. The reduction in zooplankton doesn’t allow as many fish to survive to adulthood, which, in turn, means less food for many secondary consumers like the abundant bird of prey and loon populations in and around the lakes. An even bigger concern is the excessive production of the cyanobacteria and their role in
the depletion of dissolved oxygen in bottom waters. The loss of dissolved oxygen in bottom colder waters can eliminate a cold-water fishery and lead to a warm-water fishery. This tends to reduce the top-down control by cold-water piscivores (fish-eating fishes) and promote a fishery characterized more by planktivorous (zooplankton-eating) fishes, such as white perch. As a result, there are fewer large zooplankton available to graze on the phytoplankton community, causing greater phytoplankton (including cyanobacteria) biomass and reduced water clarity. On this morning, several loons decided to swim within 15 feet of our boat, and an osprey politely told us in bird-talk to back away from its nest, high up in a pine tree looming over the pond.

Several loons, including this one, ventured within 15 feet of the boat.

Dwinal collects water samples at specific depths using a metal cylinder with caps that are spring loaded. It’s lowered to a specific depth and then the caps shut so water can be sampled at a given depth.
The work done by people like Ryan and Stephanie is important to the way human, animal, and microbiological communities function in relation to lakes and ponds. What on the surface may just seem like simple monitoring of lakes can have a massive impact on the economy of the area. I hope that the next time you look at a lake you take a moment to appreciate the vast complexity that even a relatively small body of water contains. The health of lakes and ponds has a direct and indisputable impact on us. Keeping a close eye on our freshwater resources is vital to keeping them healthy for us all.


